Background: It remains unclear whether measurable residual disease (MRD) detected by next-generation sequencing (NGS) or BCR-ABL RT-PCR is more accurate in predicting relapse and guiding clinical management in patients with Ph+ acute lymphoblastic leukemia (ALL). In this multi-center retrospective study, we examine the discordance between BCR-ABL PCR and NGS MRD to better characterize practice patterns for disease monitoring for patients with Ph+ ALL.
Methods: We included Ph+ ALL patients from five academic hospitals in the United States who were monitored with both BCR-ABL PCR and NGS-based clonoSEQ (Adaptive Biotechnologies) between 2015 and 2023. Patients were included if they had both baseline BCR-ABL PCR and NGS MRD assessments and at least 1 follow-up MRD assessment using both modalities. BCR-ABL PCR and NGS MRD results were only included if they were taken less than 1 month apart. Patient demographics, BCR-ABL isoform (p190 or p210), and IKZF1, CDK2NA, and PAX5 copy number alterations were also collected. We used frequency counts, median, and interquartile range (IQR) to describe our data. Univariable logistic regression was performed to quantify associations between diagnostic patient features and any post-diagnosis MRD discrepancy (i.e., MRD positive vs. MRD negative) between the two modalities.
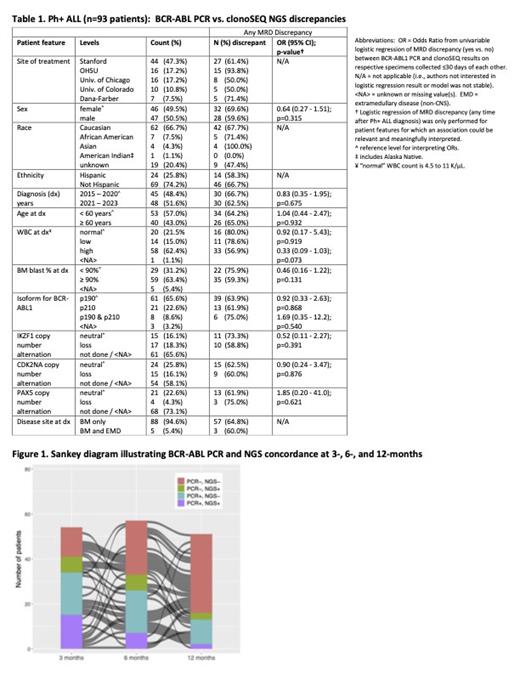
Results: 93 patients had both BCR-ABL PCR (p190 n=61 [66%], p210 n=21 [23%], both n=8 [9%]) and NGS MRD assessments. Descriptive statistics are shown in Table 1. Figure 1 is a Sankey diagram illustrating the trajectory of BCR-ABL PCR and NGS concordance at 3, 6, and 12 months following diagnosis. 60 patients (64%) had a discrepancy between qPCR and NGS at any time after Ph+ ALL diagnosis while 24 patients (26%) had a discrepancy within the first 3 months. For the 54 patients (58%) receiving transplant, 38 (70.4%) had a post-diagnosis MRD discrepancy. Among all patients, median time from diagnosis to first discrepancy was 3.9 (IQR, 2.3-7.3) months. The most common discrepancy was BCR-ABL PCR detectable / NGS undetectable (n=39, 42% of included patients). In 12 patients (20% of patients with an MRD discrepancy at any time), clinical decision making was changed due to this observed discrepancy in MRD status. While a persistently positive PCR result with negative NGS led to a change in treatment regimen for 8 of these patients (75%), one patient's positive PCR with negative NGS led to the decision to continue current therapy. Further, a positive NGS with negative PCR led to change of treatment in 3 patients. BCR-ABL isoform and copy number variation of target genes were not associated with increased risk of MRD discrepancy, although sample size was limited for the genetic alterations (Table 1).
Conclusion: We found a considerable degree of discordance between BCR-ABL PCR and NGS in Ph+ ALL patients. The most common discrepancy was PCR detectable / NGS undetectable, and in case of discordance, PCR positive results are most frequently given precedence in guiding clinical management. In the discordant patients, the presence of MRD positivity via either test drove changes to treatment rather than MRD negativity in one of the two tests. Future studies should investigate whether BCR-ABL PCR or NGS is a better predictor of outcome in Ph+ ALL.
Disclosures
Schwartz:Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy. Raman:Genentech Inc.: Consultancy. Patel:BMS: Honoraria; AbbVie: Honoraria; Kronos Bio: Research Funding; Pfizer: Research Funding. Muffly:autolus: Consultancy; astellas: Consultancy, Research Funding; kite: Consultancy, Honoraria, Research Funding; orca bio: Research Funding; bms: Research Funding; adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; jasper: Research Funding; amgen: Consultancy; pfizer: Consultancy. Leonard:Takeda: Consultancy; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Kite/Gilead: Consultancy; Pfizer: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal